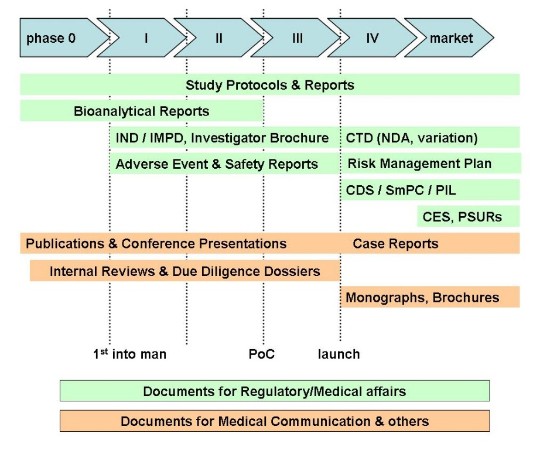
Our services comprise
- the writing from scratch
- the updating and editing
- the formatting (including cross- and hyperlinks)
- the review, proof-read, or quality control
of any kind of life science document, i.e.
- Documents for Health Authorities
- Clinical trial-related documents
- Drug safety evaluations
- Medical communications
- Scientific publications
including corresponding literature searches, data management, and statistical analyses
in compliance with the Clients’ SOPs or templates and applicable guidelines